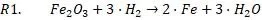ITA Explores How Hydrogen Can Improve Ore Processing in the PRIMROCK Project
by ITA - Carlos Saenz Ezquerro , Alvaro T. Latorre Molins, Alvaro Manuel Rodríguez Cambra, Carmen Alfaro Isac
As one of the leading R&D centers in Spain, the Technological Institute of Aragón (ITA) promotes technological development and innovation through its multidisciplinary team. The ITA team involved in PRIM-ROCK specializes in high-fidelity computational simulation of complex processes, particularly in multi-physics and multi-scale methodsand advanced techniques for reduced order modeling and optimization.
Within the PRIMROCK project, ITA’s contribution includes the simulation of oxide and sulphide ores in a hydrogen atmosphere at high temperature, a process known as hydrogen reduction. The aim is to obtain a comprehensive understanding of the process and assess how operating variables—such as temperature, ore-pellet size or hydrogen concentration—affect process performance.
One of the ores under study is laterite, which is used as a source of iron and nickel due to the high content of its corresponding oxides. During hydrogen reduction, hydrogen gas reacts with metal oxides, removing the oxygen from the compounds. Pure metal is left, and water is produced as a by-product. The chemical reactions are:
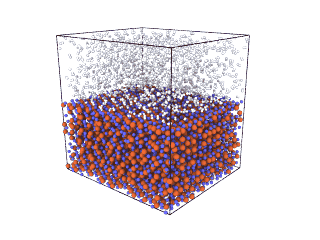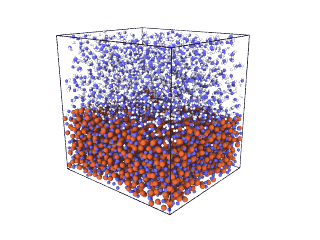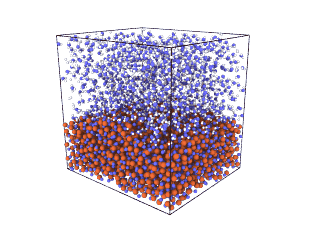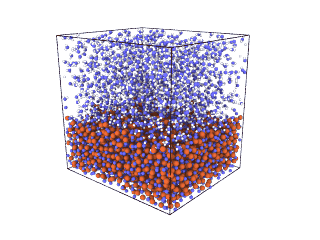
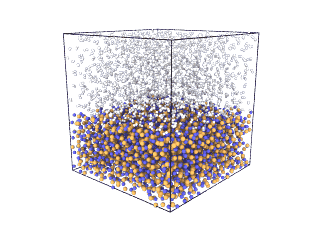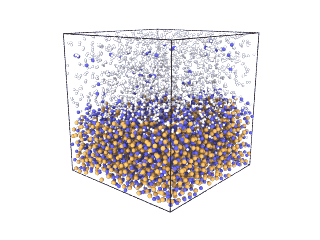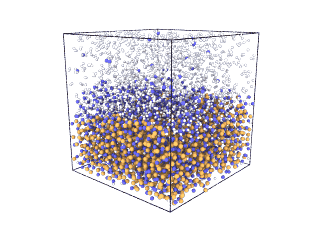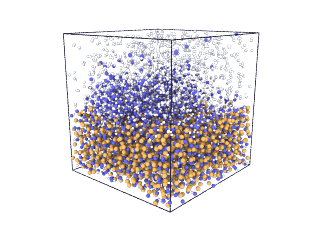
ITA has defined a multiscale methodology that connects the chemical reaction at molecular level to the metal production at particle scale. First, molecular dynamics (MD) simulations estimate the evolution of molecular interactions under different conditions and determine their kinetic parameters. In the molecular renderings (see Nickel_oxide.gif and Iron_oxide.gif), hydrogen atoms are shown as white spheres, oxygen as blue, iron as orange, and nickel as yellow. As the reduction reactions proceed, oxygen from the oxides are combined with hydrogen to form water, leaving metallic iron and nickel.
Iron oxide
Nickel oxide
Next, the molecular-scale kinetics are embedded in a CFD model of a single laterite pellet. This model explores how operating conditions, such as particle size, composition of the gas flow within the reactor (a hydrogen–nitrogen mixture), and operating temperature, influence the process performance at particle scale. The gradual formation of metallic iron and nickel in the pellet is monitored.
In the following video, the central view shows the particle behavior exposed to the H2-N2 atmosphere. As the reaction progresses, hydrogen is consumed, and water is produced. In addition, the video shows the gradual formation of Ni and Fe in the laterite cross-section over time.
Ultimately, this integrated CFD approach developed by ITA, combining molecular-level and particle-scale simulations, will be incorporated into a 3D reactor model that predicts the evolution of the industrial-scale process under different operating settings. Consequently, the reactor simulation will enable the rapid evaluation of scenarios with high precision and the identification of optimal operating conditions.
This multi-scale methodology efficiently connects molecular, particle and industrial scales, providing a detailed description of process mechanisms in each level and allowing for process optimization. This approach will be next applied to the study and process optimization of sulphide ores.